Biochemistry
Hydrogen Bonding
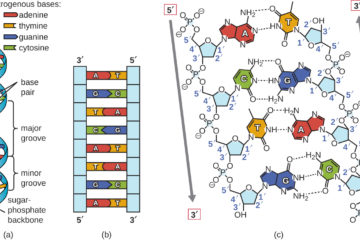
Hydrogen Bonding A particularly important type of dipole-dipole interaction is hydrogen bonding. It’s the main reason for the stability of the double-helix secondary structure of DNA, the unusual phase diagram of water, and the secondary structure of proteins in our bodies. Strictly speaking, hydrogen bonds are a special case of Read more…