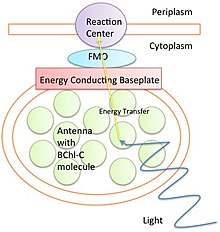
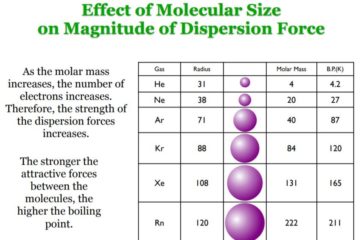
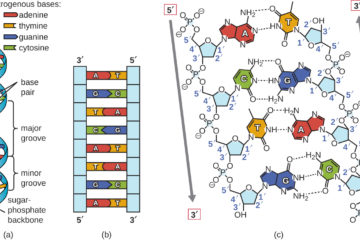
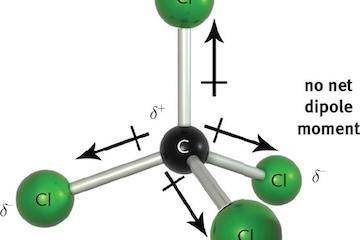
Why Dark Matter? Why not Modified Gravity?
Dark Matter vs MOND: A Comparative Overview Abstract The purpose of this post is to present a comparative overview of the dark matter hypothesis and its leading competitor: Modified Newtonian Dynamics (MOND). An abridged history of the subject is given, Read more