Introduction
In a recent article, I introduced the concept of the Gibbs energy of a chemical reaction. Before building on the content of that post, I’d like to recap its most salient points:
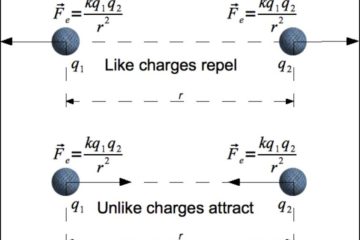
Gibbs energy change is an important and broadly applicable thermodynamic concept which provides a reliable way of determining the conditions under which specific reactions or processes will occur spontaneously.
Gibbs energy change values have been tabulated for many different reactions under standardized conditions. Since it’s a state function, various linear combinations of those values can be added and subtracted like algebraic equations to calculate the values of still other reactions.
As was the case with enthalpy, (which I covered here and here), there exist ways of adjusting those standardized values so that they still yield viable answers under non-standard reaction conditions.
The Gibbs energy of a reaction is closely tied with its equilibrium constant (K), whose numeric value represents the ratio of products to reactants at which the reaction equilibrates at a given temperature.
This provides a thermodynamic explanation for why the relative concentrations of substrates for a given reaction affects whether it will occur spontaneously (and to what extent).
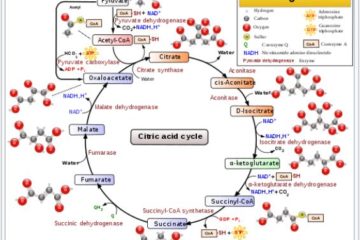
In turn, this dependence of a reaction’s spontaneity on relative substrate concentrations is one of the ways in which biological organisms naturally perform many processes that would otherwise be thermodynamically unfavorable.
My goal for this post is to use a couple of examples to illustrate how the spontaneity of some biochemical processes can be affected by relative substrate concentrations, and/or by the coupling of an endergonic (non-spontaneous) process with an endergonic (spontaneous) one. (more…)