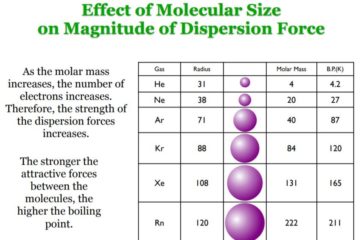
Hydrogen Bonding
A particularly important type of dipole-dipole interaction is hydrogen bonding. It’s the main reason for the stability of the double-helix secondary structure of DNA, the unusual phase diagram of water, and the secondary structure of proteins in our bodies. Strictly speaking, hydrogen bonds are a special case of dipole-dipole interactions, but they are important in so many ways that they are usually taught as separate type of interaction. Part of the reason for this is that, although they are nowhere near as strong as covalent or ionic bonds, hydrogen bonds have a slightly higher dissociation energy than other common dipole-dipole interactions.
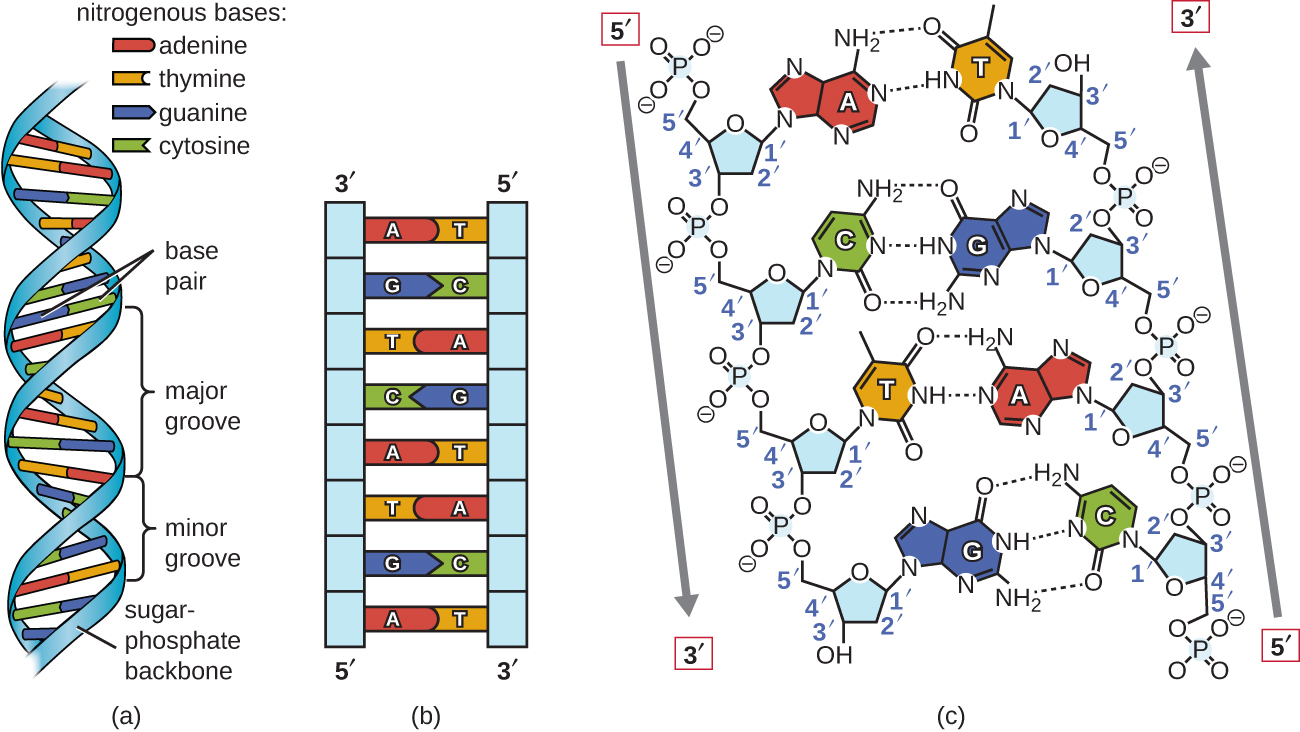
Image Source:
When many hydrogen bonds work together, they can stabilize macromolecules such as DNA, yet they aren’t so strong as to prohibit their dissociation entirely. If your DNA couldn’t be unzipped for replication or transcription at all, it’s not hard to see how that could be a problem. Part of the reason hydrogen bonds are stronger than most dipole-dipole interactions is that hydrogen’s small size permits shorter interatomic distances, and remember that electrostatic forces obey an inverse square relation.
Another way in which hydrogen bonding is particularly important in chemistry, biology, and biochemistry is the behavior of water (H2O). Oxygen is much more electronegative than hydrogen, which makes water a highly polar molecule.
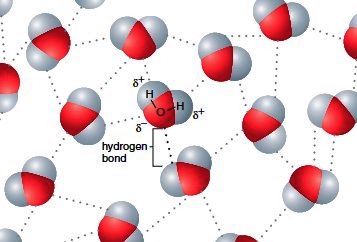
Image Source:
As depicted in the image above, the partial positive charges on the hydrogens interact strongly with the partial negative charges on the oxygen, which causes certain configurations of adjacent water molecules to be favored over others.
This property of water is responsible for certain molecules being more water soluble than others. Positively charged ions and/or the partially positively charged ends of polar molecules interact attractively with the partially negatively charged oxygens of the water molecules, whereas negatively charged ions and/or the partially negatively charged ends of polar molecules interact attractively with the partially positively charged hydrogens of the water molecules.
Return to Table of Contents:
Go to next section: Van der Waals forces.
Go to previous section: Ion-dipole interactions and hydrophilicity.