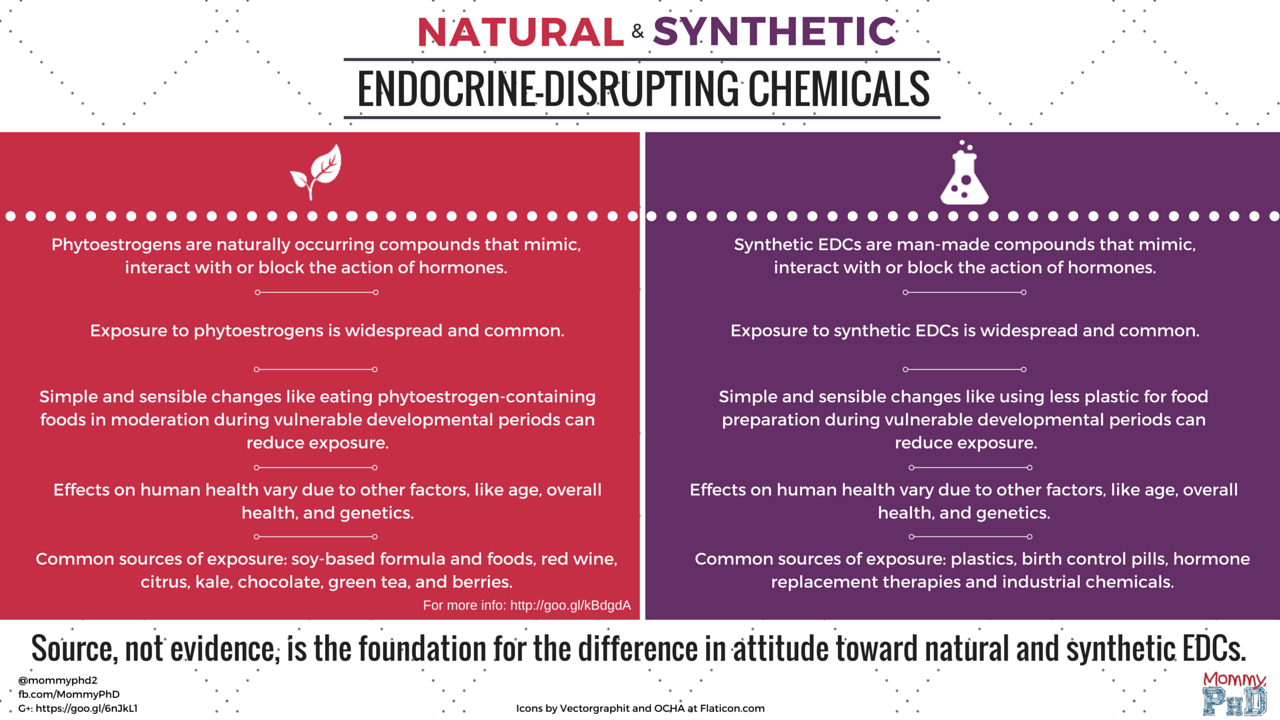
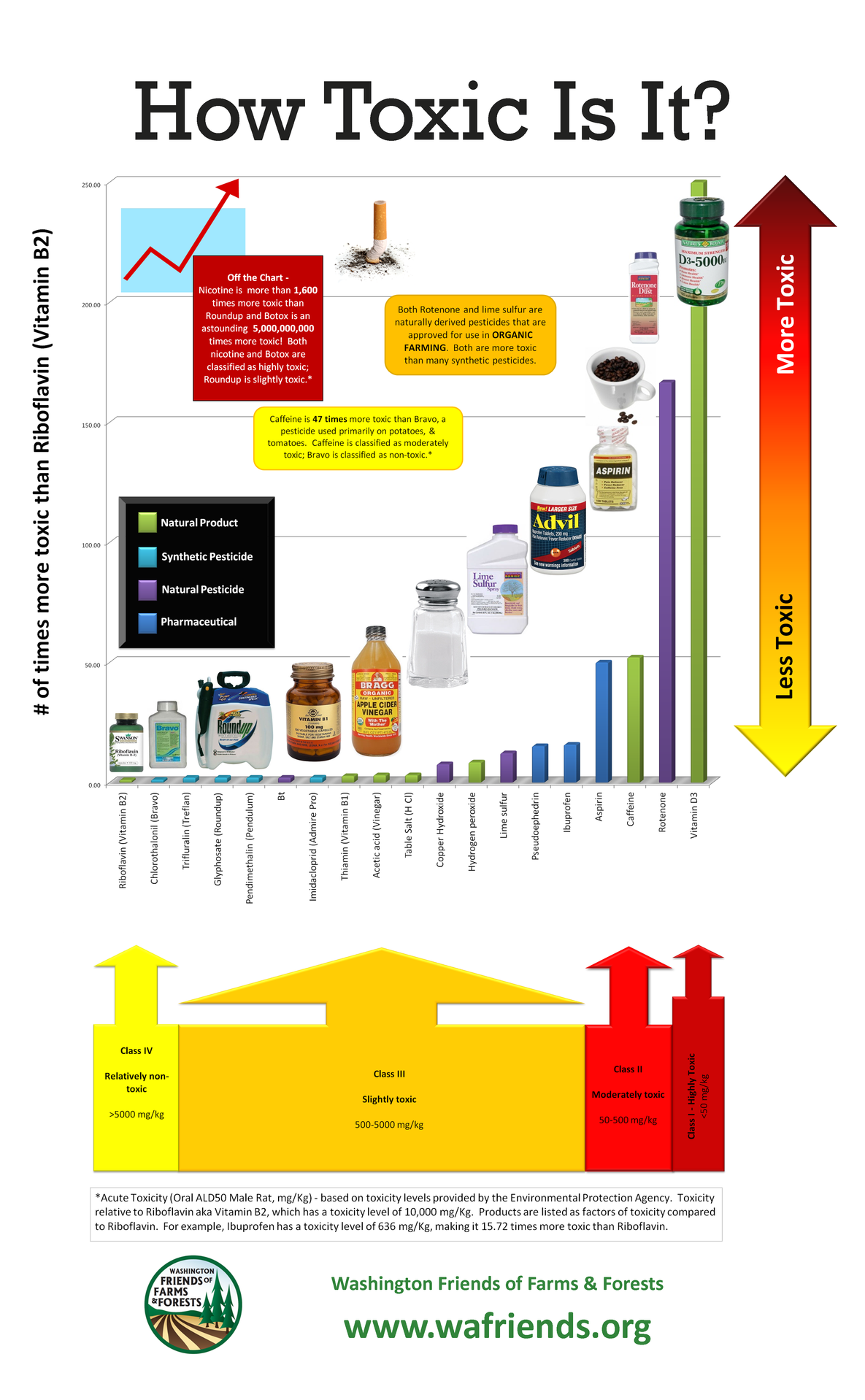
A chemical is a chemical is a chemical
A chemical’s properties are not affected by whether it was made by God, Mother Nature, evolution, or a chemist in a lab coat.
There’s a certain irony to drinking a soy latte from a BPA-free mug, but that’s not something the chemophobic fear squad seems to understand. This fear-mongering crowd would have you believe that synthetic endocrine-disrupting chemicals (EDCs) are going to be the downfall of civilization as we know it (I admit, that’s hyperbolic, but so are many of their exaggerated claims). However, in a truly perfect example of inconsistent application of the precautionary principle and selective consideration of data and data gaps, they completely overlook the potential risks of phytoestrogens, naturally occurring endocrine-disrupting chemicals found in soy and other common foods. (more…)